Custom Synthesis
Custom synthesis means the exclusive synthesis of compounds on behalf of the customer, i.e., you can order a specific molecule that is only synthesized on your request on the scale, with the purity and with the specification or methods you require.
Some of Custom Synthesis
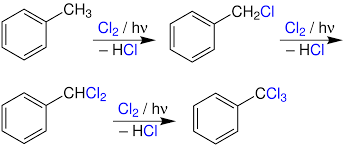
Photo Chlorination
It is a chlorination reaction that is initiated by light. Usually a C-H bond is converted to a C-Cl bond. Photochlorination is carried out on an industrial scale. The process is exothermic and proceeds as a chain reaction initiated by the homolytic cleavage of molecular chlorine into chlorine radicals by ultraviolet radiation.
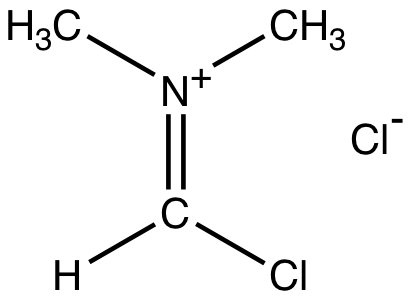
Vismeeer Hacck Reaction
Vilsmeier reagent is the active intermediate in the formylation reactions, the Vilsmeier reaction or Vilsmeier-Haack reaction that use mixtures of dimethylformamide and phosphorus oxychloride to generate the Vilsmeier reagent, which in turn attacks a nucleophilic substrate and eventually hydrolyzes to give formyl.
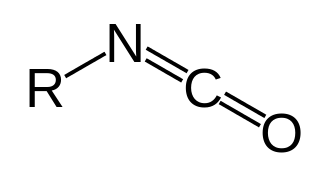
Synthesis Of Acylisocyanates
In organic chemistry, isocyanate is the functional group with the formula R−N=C=O. Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers.
Isocyanates should not be confused with cyanate esters and isocyanides, very different families of compounds. The cyanate (cyanate ester) functional group (R−O−C≡N) is arranged differently from the isocyanate group (R−N=C=O). Isocyanides have the connectivity R−N≡C, lacking the oxygen of the cyanate groups
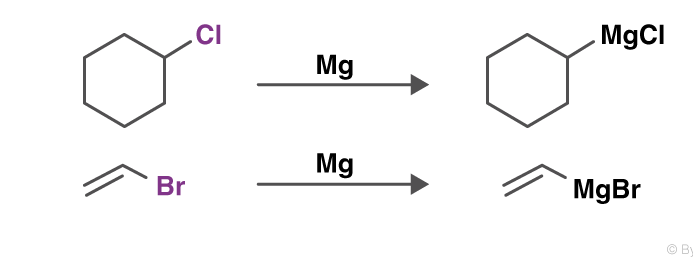
Grignard reaction
The Grignard reaction is a prominent textbook process to form carbon–carbon bonds. In this reaction, the so-called Grignard reagent, an organomagnesium species RMgX where R is an organic residue and X is a halogen (usually Cl or Br), promotes the addition of its organic residue to an electrophilic substrate.
Esterification
Esterification is the process of combining an organic acid (RCOOH) with an alcohol (ROH) to form an ester (RCOOR) and water; or a chemical reaction resulting in the formation of at least one ester product. Ester is obtained by an esterification reaction of an alcohol and a carboxylic acid.
N- Methylation
Some esters can be prepared by esterification, a reaction in which a carboxylic acid and an alcohol, heated in the presence of a mineral acid catalyst, form an ester and water: The reaction is reversible. As a specific example of an esterification reaction, butyl acetate can be made from acetic acid and 1-butanol.
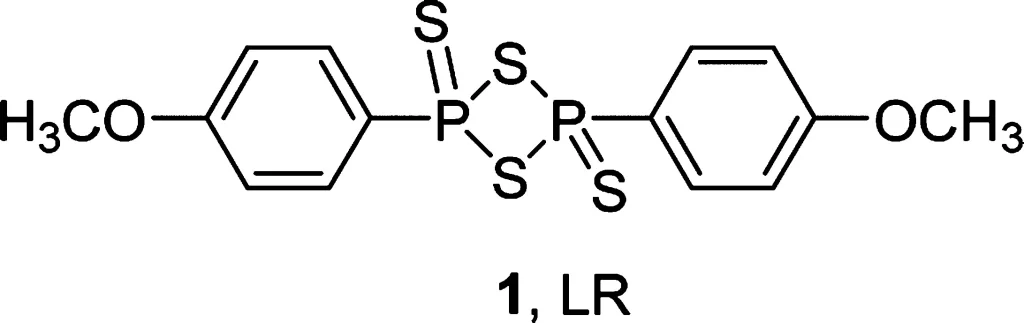
Thiation of Carbonyl Moiety
The thiocarbonyl group is widely found in a great variety of organic compounds. Thio analogues of ketones, lactones, amides, and esters are very important biological molecules, and they are widely used in medicine as therapeutic agents with a wide range of biological activities. Thiocarbonyl compounds have also been widely used in organic synthesis as precursors of organosulfur compounds and can participate in essentially the same reactions of the counterpart carbonyl derivatives, although showing, in general, a lower reactivity. Furthermore, thiocarbonyl compounds have a rich photochemistry providing excited states that lead to various photoproducts. The more expeditious way of preparing a thiocarbonyl derivative is the direct thionation of the corresponding carbonyl compound.
Phosphorus/ Sulphur Free Chlorination

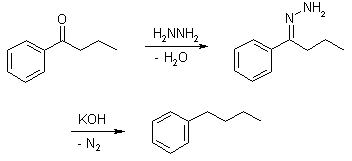
Wolff-Kishner Reduction
Wolff-Kishner reduction mechanism begins with the formation of a hydrazone anion which then releases the nitrogen molecule to form a carbanion. This carbanion then reacts with the water in the system to give a hydrocarbon. Typically, diethylene glycol is used as a solvent for this method. This reduction is an organic reaction where aldehydes and ketones are reduced to alkanes. Some carbonyl compounds are stable in strongly basic conditions, hence they can be easily reduced to alkanes (The carbon-oxygen double bond becomes two carbon-hydrogen single bonds). Although the mechanism usually begins with the condensation of hydrazine to give a hydrazone, the usage of a pre-formed hydrazone can have advantages such as reduced reaction time, reactions that proceed at room temperature or very mild reaction conditions. The pre-formed hydrazone substrates that can be used in this reduction also require different solvents and reaction temperatures.
Introduction to Sulphur
Sulphur is a non-metallic chemical element identified by the letter S. . Sulphur is a valuable commodity and integral component of the world economy used to manufacture numerous products including fertilizers and other chemicals. For a list of sulphur uses Sulphur also is a vital Nutrient for crops, animals and people.

Address : Anshul Innovative Chemistry Private Limited, Flexcel Park, C Wing, 2nd Floor,S. V. Road, Near 24 Karat Multiplex, Jogeshwari (West), Mumbai 400 102.
Useful Links
Subscribe Now
Don’t miss our future updates!
